MedWaves AveCure® Ablation System: Microwave Energy Used to Treat Lung Tumor
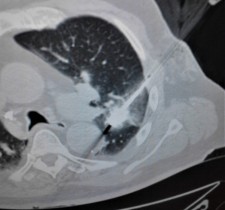
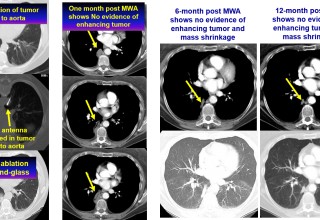
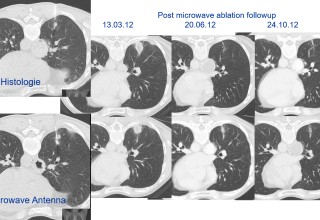
SAN DIEGO, December 4, 2018 (Newswire.com) - Doctors at National Taiwan University are successfully using microwave energy to minimally invasively treat tumors in the lung with higher local success rate of 84 percent compared to historical 67-78 percent.
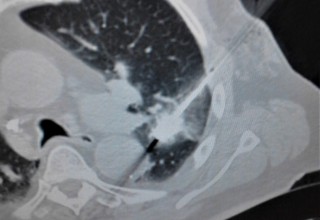
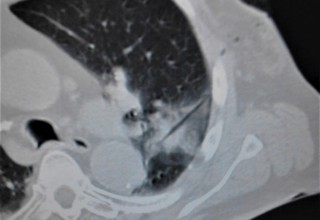
The doctors apply microwave energy to destroy tumors located both centrally and peripherally within the lung using computer tomography (CT) image guidance. The tumors include colorectal cancer, lung adenocarcinoma, thymoma, uterine leiomyosarcoma and ampullary carcinoma in origination. Microwave energy applicators are introduced into the tumors in the lung percutaneously, a minimally invasive technique. A small hole is made through the skin near the tumor location and an applicator is inserted into the lung cavity and lung towards the tumor. Once the positioning of the antenna with respect to the tumor is verified with CT image guidance, microwave energy is applied to the antenna in contact with the tumor to destroy it. The procedures usually take an hour or two depending on the complexity of tumor location and shape to achieve good antenna positioning. The microwave energy application is controlled with direct temperature feedback from the ablation antenna during the procedure to ensure safety and efficacy. Temperature feedback control prevents overheating and runaway conditions and thereby provides the extra safety net for the patients and procedures.
AveCure® microwave ablation system is successfully treating tumors in the lung using a minimally invasive technique. The microwave ablation antenna is introduced through a percutaneous chest wall incision in the skin to access the tumor past the lung cavity and patient is left with a small hole in the skin which quickly heals with almost no scar after the procedure. AveCure® system utilizes a smart antenna in either probe or catheter format and microwave energy controller to select the correct size, temperature and timer settings appropriate for safe, effective and predictable treatment.
AveCure® microwave ablation system is FDA 510(K), CE Mark and COFFEPRIS registered. The MedWaves AveCure® Ablation System is intended for use in percutaneous, laparoscopic and intraoperative coagulation-ablation of soft tissue.
The MedWaves Ablation System is not intended for use in cardiac procedures.
MedWaves Inc. is a privately held company with headquarters and manufacturing in San Diego, California. www.medwaves.com, Tel: +1 760-807-1000, tedormsby@avecure.com
Source: MedWaves Inc.