Microwave Energy Used to Successfully Treat Benign Breast Tumors
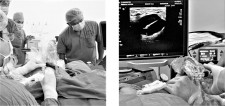
SAN DIEGO, May 6, 2020 (Newswire.com) - In a recent World Journal of Surgery and Surgical Research publication, doctors Huynh Quang Khanh and Tran Quyet Tien at Department of Breast, Cho Ray Hospital, Ho Chi Minh City, Vietnam shared the results of treating 41 patients with 64 benign breast tumors, and cysts from 2018 to 2019. Patients underwent microwave ablation (MWA) using MedWaves AveCure Microwaves Ablation system developed and manufactured in San Diego, CA USA. Results: 100% rated excellent for post-ablation aesthetics, and there were no side effects, relapse, or malignancies during follow-up. The study concluded MWA of benign breast tumor, and cyst is feasible and highly efficient. Symptoms and lesion sizes were significantly reduced, with fast resilience (recovery of shape) and aesthetics preservation.
AveCure® microwave ablation system is FDA 510(K), CE Mark and COFFEPRIS registered. The MedWaves AveCure® Ablation System is intended for use in percutaneous, laparoscopic, and intraoperative coagulation-ablation of soft tissue.
The MedWaves Ablation System is not intended for use in cardiac procedures.
MedWaves, Inc is a privately held company with headquarter and manufacturing in San Diego, CA. www.medwaves.com, tedormsby@avecure.com mobile: (760) 807-1000
Source: MedWaves, Inc.
