MedWaves AveCure® Ablation System: Microwave Energy Used to Successfully Treat Brain Tumor
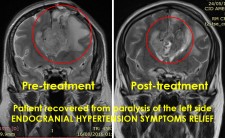
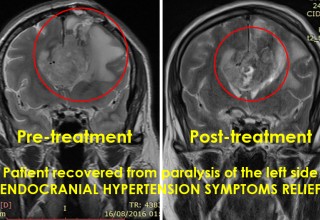
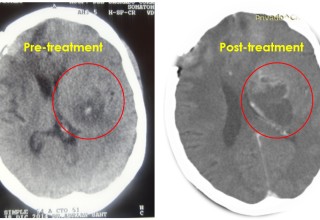
SAN DIEGO, November 19, 2018 (Newswire.com) - Doctors at a major hospital in Guadalajara, Mexico, is successfully utilizing microwave energy to treat inoperable brain tumor patients to relieve pain, reverse neurological deficits, restore quality of life and improve survival periods. Until recently, patients with brain tumors have limited choices if the tumor continues to grow or reoccurs locally following a surgical or radiation therapy. Patients who receive these traditional therapies and fail are usually left with no additional treatment options due to further impairment from the procedures.
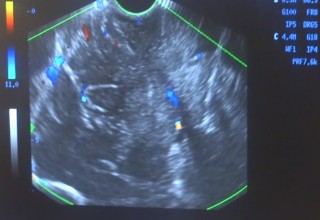
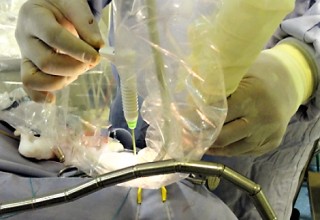
The doctors applied microwave energy ablation as either adjunct or standalone technique during minimally invasive procedures to treat patients who have run out of options. The microwave energy, when used in adjunct to microsurgery, allows for more complete removal of tumors while reducing collateral damage. This results in quicker and improved recoveries. The microwave energy when used standalone through a burr-hole and/or natural opening, allows for treatment of non-resectable tumors in one session or multiple sessions while preserving neurological functions, resulting in faster and long-term options for complex brain tumors. The doctors restored quality of life and improved survival periods for these patients with very little therapeutic options. The microwave energy application is controlled with direct temperature feedback from the ablation antenna during the procedure to ensure safety and efficacy, and ultrasound imaging is used to position the microwave antenna and to observe microwave field interaction with the tumors.
AveCure® microwave ablation system is successfully used by doctors in Guadalajara, Mexico, to treat and relieve symptoms from brain tumor patients. The system is used as an adjunct in microsurgery or as the standalone technique to cook the tumor from inside. The procedure is minimally invasive: a small flap, an access burr-hole or normal opening to introduce a microwave antenna using ultrasound imaging guidance to gain accurate antenna position. AveCure® system utilizes a smart antenna in either probe or catheter format and microwave energy controller to select the correct size, temperature and timer settings appropriate for safe, effective and predictable treatment.
AveCure® microwave ablation system is FDA 510(K), CE Mark and COFFEPRIS registered. The MedWaves AveCure® Ablation System is intended for use in percutaneous, laparoscopic and intraoperative coagulation-ablation of soft tissue.
The MedWaves Ablation System is not intended for use in cardiac procedures.
MedWaves Inc is a privately held company with headquarters and manufacturing in San Diego, California. www.medwaves.com, tedormsby@yahoo.com, Mobile: 760-807-1000
Source: MedWaves Inc.