Bridge Clinical Research Partners With Stanford Precision Health for Ethnic and Racial Equity to Launch New Study Involving Sickle Cell Disease Patients and Precision Health

OAKLAND, Calif., December 7, 2018 (Newswire.com) - Bridge Clinical Research and Stanford Precision Health for Ethnic and Racial Equity, SPHERE, were recently granted IRB approval to launch a new study titled “The Impact of Physician Race on Sickle Cell Disease Patients’ Understanding of Precision Health and Willingness to Participate in Precision Health Research.” The study is set to take place early next year, 2019.
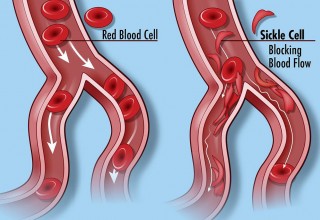
Sickle cell disease (SCD) is a hereditary blood disorder characterized by sickle-shaped red blood cells. SCD is a chronic, life-long, debilitating disease with many forms that can range in clinical severity from asymptomatic to life-threatening. It occurs predominantly in individuals of African descent and is also prevalent throughout the Mediterranean, the Middle East and parts of India, the Caribbean, and South and Central America. SCD is a major public health concern.
There has been a surge of traditional and targeted therapies by companies, with many of these being potential new treatments leveraging many aspects of precision health. The scientific, patient and SCD advocacy communities are enthused about the onset of technology to develop new treatments, but there is a disconnect between the availability of promising therapies and historical unwillingness of the minority SCD community to participate in research. While there are multiple causes for low participation rates, one primary issue is the mistrust of medical professionals by the African-American community. Therefore, this study will examine how patient perspectives on precision health research are influenced by the race of the physician or researcher presenting the concept of precision health in an effort to address the ethical issue of justice and the historical underrepresentation of groups in biomedical research.
The primary collection of data for this study will be through a randomized controlled trial. A survey tool will be used to determine if participants are willing to engage in precision health research based on various scenarios and physician photos. There will be questions generated to understand what factors may increase a person’s willingness to participate in precision health research. Finally, a post-survey will be given to all participants to again determine their awareness of precision health and determine the retention of basic information based on what was presented.
Upon completion of this research, Bridge Clinical Research and SPHERE are expected to assess actual enrollment of SCD patients in precision health research. Additionally, there will be other natural opportunities for further study, including introducing actual physicians into a study and expanding the eligibility criteria to include the pediatric population.
For more information regarding this study, please visit www.bridgeclinical.com or contact Bridge Clinical Research at riccesha.hattin@bridgeclinical.com.
Source: Bridge Clinical Research