2021 INS Standards Recognize SecurePortIV® Tissue Adhesive as Standard of Care
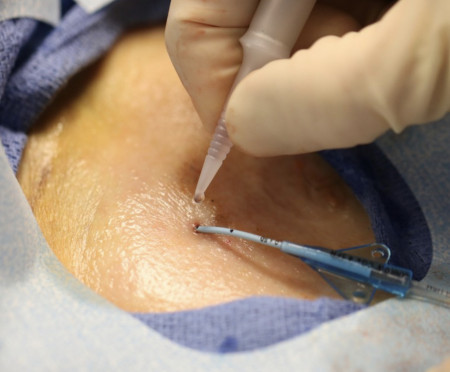
WYOMISSING, Pa., January 19, 2021 (Newswire.com) - Adhezion Biomedical, LLC is excited to announce that the just-published 2021 Infusion Therapy Standards of Practice (INS Standards) recognizes tissue adhesive as a standard of care for all vascular access device (VAD) care and management. Adhezion Biomedical's SecurePortIV® is the only tissue adhesive cleared for this indication by the US FDA.
The INS Standards recognize this new technology for a broad set of benefits including:
- Stabilizing and securing VADs to reduce dislodgement and device failure
- Sealing the insertion site creating a barrier to microorganism growth
- Reducing the risk of catheter-associated infection
- Providing immediate hemostasis at the insertion site to reduce dressing changes
The INS Standards are a set of evidence-based practice recommendations that are published every five years, where adherence to the standards promotes consistency in patient care guides clinical decision-making and enhances competency. Each standard is evaluated for the level of evidence supporting that recommendation. Standards with a large body of evidence are identified as Level I or Level II and carry strong practice recommendations. The evidence levels for the use of tissue adhesive are predominantly Level I or Level II.
This new standard further supports what many leading hospitals in the US have shared with their clinical peers in presentations at the 2019 and 2020 Association for Vascular Access Scientific Meetings including: Mayo Clinic, Beaumont Health, Moses Cone Health System, St. Joseph's Medical Center, University of Pittsburgh Medical Center, Texas Health Huguley Hospital, WakeMed Health, Loma Linda University Medical Center, Glens Falls Hospital, Children's Hospital of Orange County, Children's Hospital of Philadelphia, Rady Children's, Cook Children's Hospital, Driscoll Children's Hospital, Children's Mercy in Kansas City, Nationwide Children's, Dayton Children's Hospital, Boston Children's Hospital, and UCSF Benioff Children's Hospital - Oakland.
Marcia Wise, Adhezion's Medical Director for Vascular Access, said, "It is so exciting to see how this technology, which has been used for years to close wounds, is now being used to secure, seal and protect our vascular access insertion sites. Rarely in my 30+ year career in vascular access have I observed a product move from initial trials to being accepted as a standard of care so quickly. This is a direct result of strong data published and presented by the clinicians listed above. I remember meeting the Adhezion team in 2017 and seeing SecurePortIV® adhesive for the first time. I told the team that I anticipated that this product would become a standard of care within five years. I was wrong! It only took four!"
Pete Molinaro, Adhezion's CEO and Chairman, said, "It is gratifying for us to see SecurePortIV® recognized for its valuable clinical benefits in such a short time since its launch and to watch it become a disruptive and essential technology in the field of vascular access."
About Adhezion Biomedical LLC: Adhezion Biomedical is a privately held medical device company based in Wyomissing, PA. Adhezion is committed to developing and manufacturing medical cyanoacrylate products used in the treatment of wound closure, wound management, intravenous device securement and infection prevention. To learn more about Adhezion and its products, please visit www.adhezion.com.
Contacts:
Investors
Pete Molinaro
+1.610.741.6049
pmolinaro@adhezion.com
Media
Rachel Brizek
+1.610.750.5917
rbrizek@adhezion.com
Source: Adhezion Biomedical, LLC
