Vesalius Cardiovascular Reaches a New and Advanced Pre-Clinical Milestone
VANCOUVER, British Columbia, April 21, 2021 (Newswire.com) - Vesalius Cardiovascular Inc. (VCI), a Canadian MedTech company developing a Transfemoral Mitral Valve Repair (TMVr) solution, announced today the achievement of a new pre-clinical milestone with its 180-day chronic animal study. The predetermined endpoints of the study were the feasibility and durability of VCI's papillary muscle anchoring device. Both endpoints were confirmed at the 180-day mark.
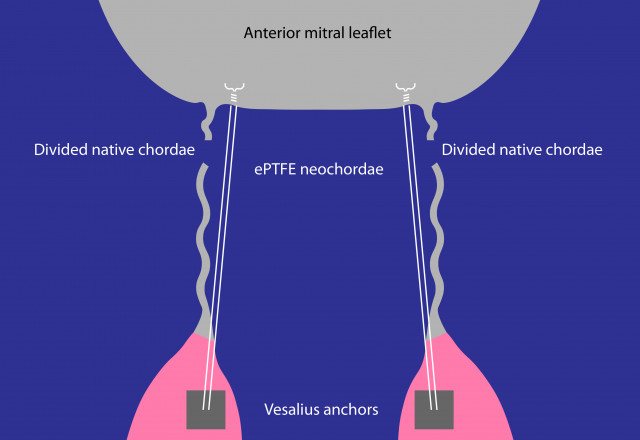
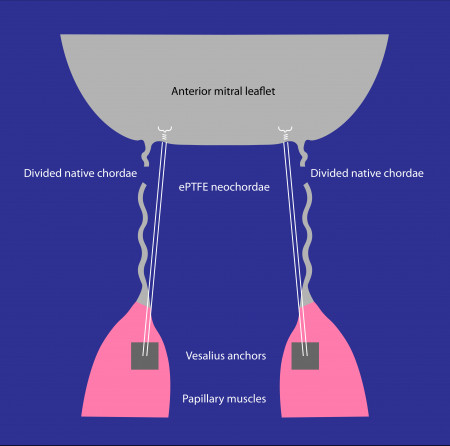
Dr. Peter Skarsgard, veteran cardiac surgeon and VCI's Chief Scientific Officer, commented, "Our catheter-based solution can reproduce surgical mitral valve repair, but without the burden of the operation. We designed and developed a TMVr solution that is based upon proven surgical principles and material. Our concept is derived from the clinically proven chordal replacement technique and uses a papillary muscle anchoring device. In this specific study, our anchors replaced the native chordae attachments to the papillary muscles and are successfully carrying the load of the functioning anterior leaflet, preventing mitral regurgitation. This is proof that our anchoring device can durably reproduce a surgically placed suture in the papillary muscle."
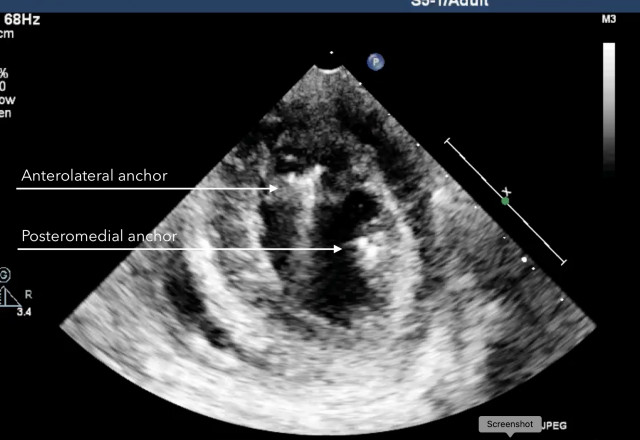
Echocardiographic images were obtained during the procedure and, subsequently, at seven, 30 and 180 days by Dr. Christopher Durkin, a cardiovascular anaesthesiologist, echocardiographer, and VCI's clinical advisor, who added, "Imaging at 180 days confirms the absence of mitral regurgitation and, more importantly, the absence of anchor migration, proving that VCI's anchors are acting exactly like the native chordae attachments."
"This milestone is a major step in our development plan," added Vincent Ledoux, VCI's Chief Operating Officer. "Despite the pandemic, we were able to maintain our timeline intact thanks to the firepower of eight experienced biomedical engineers, continuously translating decades of cardiovascular expertise into a TMVr solution. We have transformed a complex operation (open heart) that uses a simple repair tool (sutures) into a sophisticated device with a simple delivery procedure."
Dr. Jacqueline Saw, structural interventional cardiologist and VCI's clinical advisor, noted, "The VCI solution is comprehensive mitral valve repair, delivered with a catheter. The device design leads to remarkable simplification and standardization of the delivery procedure, from case to case, with no compromise on effectiveness. This optimizes user needs, for both patient and provider."
"Our unique solution is monopolistic in that we can treat the full spectrum of Degenerative Mitral Regurgitation, whether simple or complex, with or without annular dilation, using adapted chordal replacement and annuloplasty concepts in one device. No other solution can offer that," said Dr. Skarsgard, adding, "Simply said, in any size valve, our device can treat any expression of degenerative disease - it is a one-size-fits-all solution."
To date, VCI has raised $5 million from a solid base of investors and early believers and is expecting to reach the First-In-Human stage by mid-2022.
Source: Vesalius Cardiovascular