The Sounds of Nevi: Diagnosing Skin Cancer More Accurately With a New Deep Learning Technology and Telemedicine
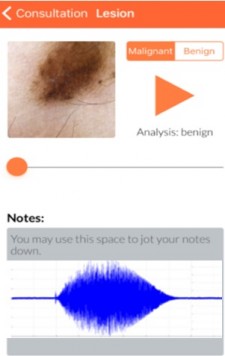
BOSTON, February 27, 2019 (Newswire.com) - Bostel Technologies, a clinical-stage healthcare company, has developed a unique technology for skin cancer diagnosis by using sonification, which is utilizing images converted to sound. Proprietary patented techniques allow all physicians to diagnose skin cancer on site by telemedicine as an AI-powered clinical decision support device.
Skin cancer, the most common cancer in the U.S., affects 5 million patients annually. The deadliest form, melanoma, is expected to be diagnosed in 96,000 people, with 7,200 people dying from the disease in 2019. Early detection of melanoma is a clinical window of opportunity that can provide an almost 100 percent rate of survival. Today, clinical accuracy in the detection of skin cancer has limited diagnostic accuracy due to the complexity of visual inputs embedded in a dermoscopy image, and its dependency on physician skills.
For example, a 23-year-old female patient approached a dermatologist in 2018. She had a small new mole on her thigh. It was diagnosed as benign. She then consulted a second dermatologist who used a computer vision system, which again categorized the mole as benign. Stubbornly, she asked for a third opinion. A trained dermatologist used dermoscopy, which identified it as malignant melanoma. It was biopsied and diagnosed by histopathology as malignant melanoma in situ.
"This circumstance is not exceptional and represents the difficulty in diagnosing melanoma at its start, during the gold period of excision," says Dr. A. Dascalu, dermatologist and chief scientific officer at Bostel Technologies.
Bostel Technologies uses a regular dermoscopic image, which is transmitted and processed by cloud computing through a convolutional neural network - a class of deep learning. The image is further deciphered by a sonification technique, which amplifies the detection accuracy of skin cancer. A recommendation to excise or not is conveyed to the physician within seconds, achieving a superhuman accuracy. This new technology was developed by a team including clinicians and experts with backgrounds in sonification and computer vision.
A clinical study published in Lancet EBioMed successfully field tested and validated the sonification technology. Sixty-three patients were prospectively identified and biopsied, 28 of them identified as skin cancer malignancy. The sonification met its primary aims of sensitivity (86 percent) and specificity (69 percent). By adding an additional layer of visual analysis through an additional deep learning system, both negative and positive predictive values were at 89 percent.
“Bostel Technologies’ combined two-layer diagnostic platform is expected to accurately assist clinical decisions by increasing their ability to diagnose skin cancer, especially melanoma,” adds Dr. Dascalu. “Most of the global R&D is directed at treatment and not prevention of melanoma. In an age of new biologic treatments, which strain healthcare budgets, the focus should be redirected to preventive medicine. Bostel’s telemedicine technology will increase the accuracy of diagnosis and decrease unnecessary skin excision, de-escalating the current trend of over-diagnosis of skin cancer.”
For more information about the company and this new technology, visit BostelTechnologies.com.
About Bostel Technologies
Bostel Technologies is a clinical-stage healthcare company that has developed a unique technology for skin cancer diagnosis by using sonification. For more information, visit BostelTechnologies.com.
Media Contact
Harry Keegan
Bostel Technologies, LLC
(781) 347-9690
hkeegan@pyrrhicv.com
Source: Bostel Technologies