PolarSeal Announces Wearable Medical Device Solutions in the US to Change Outcomes for 88 Million People at Risk for Type 2 Diabetes
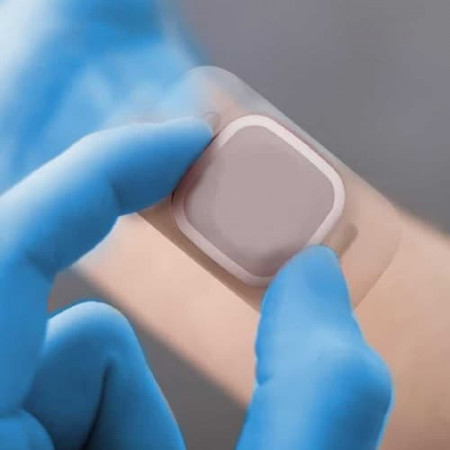
LONDON, April 25, 2022 (Newswire.com) - PolarSeal launches advanced in-house converting capabilities for adhesive tapes and medical grade materials to co-design and manufacture wearable medical devices in the US, and deliver innovative stick-to-skin solutions.
PolarSeal ensures adhesives and product materials are skin-friendly, absorbent, repositionable, and sterile. Breathable or non-breathable constructions ensure products are functional and comfortable.
Quality, cost-effective wearable medical devices enable better diagnosis and treatment of those diagnosed with, or at risk for, diabetes by pairing advanced technology with stick-to-skin adhesives for effective patient monitoring. They save and transmit data used by other medical devices or for analysis by medical professionals.
With 26.9 million Americans (CDC National Diabetes Statistics Report, 2020) of all ages (8.2% of the population) having diagnosed diabetes and 88 million more Americans at risk for Type 2 diabetes (CDC Newsroom, 2021), wearable medical device technology for early detection and monitoring of diabetes can significantly change patient outcomes.
86% of 500 patients using prescribed wearable medical devices (Business Wire) agreed that their devices improved their health, quality of life, and enabled doctors to provide higher quality care.
North America occupies the largest market share for wearable medical devices with ongoing expansion, primarily due to the increase in obesity nationwide. Demand for patient monitoring devices for the geriatric population has also increased, as wearable devices are relied upon to diagnose and treat issues associated with this group. The global wearable healthcare devices market is expected to reach USD 93.48 billion by 2030 (Research and Markets).
Medical professionals rely on wearable device solutions to pair advanced technology, high-quality materials and innovative adhesives for effective patient monitoring. Patients on the other hand want a comfortable, easy-to-use and minimally obtrusive device that doesn't interfere with daily life.
Shaun Kemp (PolarSeal):
"Wearable medical device solutions are critical in providing supply to a growing market. Working with suppliers such as 3M, PolarSeal Tapes and Conversions combines advanced in-house converting capabilities with access to medical-grade material and skin-friendly adhesives to co-design, develop, and manufacture user-friendly and bespoke wearable medical device solutions"
About PolarSeal
PolarSeal produces individual high-quality components used to develop wearable medical devices, and provides end-to-end manufacturing solutions - from concept development to packaging certified products with optimal functionality.
Extensive experience in Tape Converting and Adhesives allows us to work with medical device companies through the design and product testing process to identify the correct adhesive and materials for your device, considering the application, wear time, size and weight, and the area of application.
Additional information about PolarSeal Tapes and Conversions Ltd is accessible: https://www.polarseal.net/contact-us
Contact: Sam Power
Cell: +44 1252 726 000
PolarSeal
Webpage: polarseal.net
Email: enquiries@polarseal.net
Facebook: @polarsealuk
LinkedIn: linkedin.com/company/polarseal
Source: PolarSeal
