Physicians for Informed Consent Releases New Educational Documents; States 'DTaP Vaccine Has Not Been Proven Safer Than Diphtheria, Tetanus and Pertussis'
NEWPORT BEACH, Calif., November 21, 2023 (Newswire.com) - Physicians for Informed Consent (PIC) has introduced three new educational documents to assist parents in comparing the risks of diphtheria, tetanus and pertussis (whooping cough) to the risks of the DTaP (diphtheria, tetanus and pertussis) vaccine. The documents provide important data compiled from sources such as the National Center for Health Statistics and add to the organization’s growing collection of infectious disease and vaccine resources.
New documents in the collection include:
- “Diphtheria – Disease Information Statement (DIS)”
- “Tetanus – Disease Information Statement (DIS)”
- “DTaP – Vaccine Risk Statement (VRS)”
The “Pertussis (Whooping Cough) – Disease Information Statement (DIS)” was published earlier this year.
“I remember being a new mom and worrying about childhood infections and SIDS,” said Dr. Shira Miller, founder and president of Physicians for Informed Consent. “Back then it took so long to dig up the relevant scientific data to weigh the risk of the infection versus the risk of the vaccine. Today, again, we provide more educational resources that make it a lot easier for parents to do the math."
Key facts from the documents include the following:
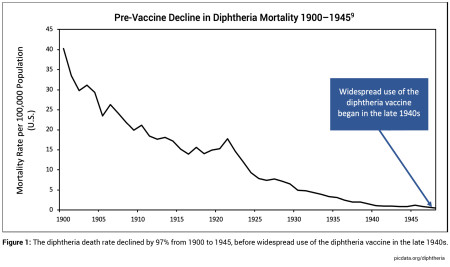
- In the modern era, it is rare to contract a fatal case of diphtheria, tetanus or pertussis in the United States. Between 1900 and 1945, before widespread use of the DTP vaccine, the mortality rate of diphtheria, tetanus, and pertussis dropped significantly (by 97%, 79% and 92% respectively) due to advancements in living conditions, sanitation, nutrition, and health care.
- In the absence of mass vaccination, for children under age 10, the annual risk of fatal diphtheria, tetanus, and pertussis respectively is 1 in 1.7 million (or 0.00006%), 1 in 784,000 (or 0.0001%), and 1 in 323,000 (or 0.0003%) — and the cumulative annual risk of a fatal case of any of those diseases is about 1 in 200,000 (or 0.0005%).
-
DTaP is a descendant of the DTP vaccine, which was introduced in 1948; it contains aluminum, a neurotoxin. The DTaP vaccine does not prevent asymptomatic infection or the spread of diphtheria or pertussis, and it has no effect on the transmission of tetanus because tetanus is not contagious.
- The Institute of Medicine has not ruled out the possibility that DTaP vaccination can lead to neurological disorders (e.g., encephalitis, infantile spasms, ataxia, autism, transverse myelitis, optic neuritis, multiple sclerosis, Guillain-Barré syndrome, and Bell’s palsy), autoimmune diseases (e.g., chronic urticaria, serum sickness, and arthropathy), myocarditis, and sudden infant death syndrome.
- The manufacturer’s package insert states that the DTaP vaccine has “not been evaluated for carcinogenic or mutagenic potential or impairment of fertility.”
- The DTaP vaccine has not been proven safer than diphtheria, tetanus, and pertussis.
To read the new educational materials on diphtheria, tetanus, pertussis, and the DTaP vaccine, visit physiciansforinformedconsent.org/dtap.
Source: Physicians for Informed Consent
