Novartis and THREAD Join Forces to Launch FocalView App, Virtualizing Ophthalmic Clinical Trials to Allow Patients to Participate in Clinical Research Remotely

- THREAD engineers groundbreaking FocalView vision tests that aim to enable digital endpoint capture
- With an open-source platform, Novartis is making FocalView vision tests freely available to the scientific community
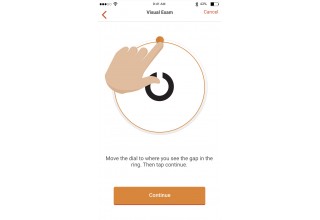
TUSTIN, Calif., May 3, 2018 (Newswire.com) - Novartis scientists teamed up with THREAD to engineer its FocalView app, an ophthalmic digital research technology. FocalView aims to allow researchers to track disease progression by collecting real-time, self-reported data directly from eligible, consented patients. FocalView deploys innovative new visual acuity and contrasts sensitivity measurements in an effort to develop potential validated instruments for future studies.
FocalView is an observational registry that is designed to help patients complete mobile consent, ePRO and conduct various assessments, gaining feedback on their visual function, including changes over time. This app could also provide researchers with a greater volume of real-world, device-reported and patient-reported data, creating more flexible and accessible clinical research designs.
There is too much subjectivity in research data collection. These visual acuity and contrast sensitivity tests are a perfect example of our interest in engineering validated objective measures for use in clinical trials.
John Reites, THREAD Chief Product Officer
"Because patients with eye diseases are often not as mobile, FocalView has the potential to offer tremendous benefit for the ophthalmic community and for researchers looking to develop better treatments for these patients," said Dr. Mark Bullimore, medical advisor for the creation of FocalView and dean of the Southern California College of Optometry, Marshall B. Ketchum University. "Collating validated patient-reported outcomes in clinical trial research is no longer a nice-to-have. This kind of data is fast becoming a critical element of research and development because it offers a better reflection of real-world patient experiences, fosters better patient compliance and provides researchers with richer and more accurate data points."
“There is too much subjectivity in research data collection. These visual acuity and contrast sensitivity tests are a perfect example of our interest in engineering validated objective measures for use in clinical trials,” said John Reites, THREAD chief product officer. "It’s exciting to see Novartis innovatively conduct remote research studies through the launch of FocalView. Their efforts with FocalView are advancing patient engagement, remote research and virtual trial approaches for our industry.”
FocalView is now available for download from the App Store in the U.S. Consent to contribute to research data will be required before a user can interact with the tool. Novartis is planning to launch in additional markets in the future.
About THREAD
From ePRO to Virtual Trials and everything in between, THREAD is a full-featured, proprietary platform enabling biopharma, CROs, and academic researchers to capture data from patients in between or in lieu of clinic visits. THREAD provides the features researchers need to design, launch and provide proper oversight of remotely captured data with patients and sites, including eConsent, ePRO, eDROs, medical devices, surveys, notifications, telehealth, and more.
For more information about THREAD, visit www.THREADresearch.com.
Media Contact:
John Reites
Phone: 919.455.3949
Email: info@threadresearch.com
Source: THREAD