ISS' Wireless Left-Heart Implantable Hemodynamic Monitoring System for Patients With Congestive Heart Failure: Exhibition at HFSA 19th Annual Scientific Meeting
ISS will exhibit its wireless left-heart implantable hemodynamic monitoring system for long-term management of congestive heart failure patients at the HFSA 19th Annual Scientific Meeting (September 26-29, 2015) in National Harbor, Maryland, USA.
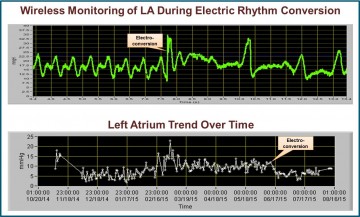
Ypsilanti, MI, September 22, 2015 (Newswire.com) - Integrated Sensing Systems, Inc. (ISS), a medical device company pioneering a micro-electromechanical MEMS-based wireless implantable pressure sensor, announced today the conclusion of a 2-year clinical study in Sweden for its miniature, wireless, left-heart, implantable, hemodynamic monitor (IHM). A single center, randomized (1:1), controlled study of 40 Swedish New York Heart Association (NYHA) Class III and IV congestive heart failure patients were implanted with the Titan™ Wireless Implantable Hemodynamic Monitoring System (WIHM) to evaluate its safety and efficacy in the management of congestive heart failure disease. The Titan™ IHM system will be exhibited in booth number 716 at the 19th Annual Heart Failure Society of America Conference 2015, 26 September – 29 September in National Harbor, Maryland, USA. The results of this study, which met its primary and secondary endpoints, continue to substantiate the high safety profile and effectiveness of the Titan™ WIHM as a viable pressure monitoring system for the management of patients with congestive heart failure, arrhythmia, and structural hearts diseases. The very compelling data from these Swedish patients demonstrates the clear promise of this wireless, left atrium pressure monitoring technology and is consistent with previous data reported from the United States.
The primary endpoints consisted of (a) the Titan™ WIHM system having a high safety profile during implantation, post-op ICU stay, one, three and six month follow-ups, and (b) the surgical implantation technique being safely used in human subjects. The secondary endpoints consisted of the Titan™ WIHM system (a) having the ability to deliver detailed pressure curves and (b) being highly functional in the great majority of occasions during OR, ICU and during one, three and six month follow-ups. Both primary and secondary endpoints were successfully met. The efficacy endpoints are also very encouraging.
Compared to the control, patients with the Titan™ IHM sensor showed shorter duration in hypotension, anuria and oliguria during ICU, and improvement in CHF left ventricle function, symptoms and rate of re-hospitalization. Due to instantaneous filling pressure monitoring, patients with the sensor further benefited from rapid effective tailoring and adjustment of medications, early detections of different types of arrhythmias, in particular fibrillation episodes, detection and management of mitral regurgitation, better management of hypertension, and early warning of the dangerous onset of tamponade.
“The conclusion of the study is a major milestone for ISS,” said Dr. Nader Najafi, President and CEO of ISS. “We are highly encouraged by the compelling and promising results for the Titan™ WIHM System and we look forward to releasing the detailed results this winter.”
About Integrated Sensing Systems, Inc.: ISS is a leader in advanced MEMS technologies for design and manufacturing of medical devices. Founded in 1995, ISS is one of the oldest independent medical MEMS companies in the United States. ISS operates a comprehensive, state-of-the-art MEMS fabrication facility located near Ann Arbor, Michigan. ISS is currently certified for ISO 9001:2008, EN13980:2002 for it ATEX (intrinsically safe products), and ISO13485:2003 standard for Class III medical devices. ISS is a vertically integrated company dedicated to developing and manufacturing system-level products based on MEMS technology (MEMS Inside), please visit: http://mems-iss.com/titan-wihm/
Source: Integrated Sensing Systems, Inc. (ISS)
Contact:
Nader Najafi, Ph.D.
Integrated Sensing Systems Inc. (ISS)
391 Airport Industrial Dr., Ypsilanti, MI 48198
Tel: (734) 547-9896 Ext. 103
Fax: (734) 547-9964
Email: nader@mems-iss.com